Medical
Cutting-edge 'peptide' shows promise in stopping breast cancer metastasis!
It is estimated that ninety percent of deaths from breast cancer are caused by complications from metastasis, so how to effectively stop breast cancer metastasis has long been a hot topic in cancer research.

It is estimated that ninety percent of deaths from breast cancer are due to complications from metastasis, so how to effectively stop breast cancer metastasis has long been a hot topic in cancer research.
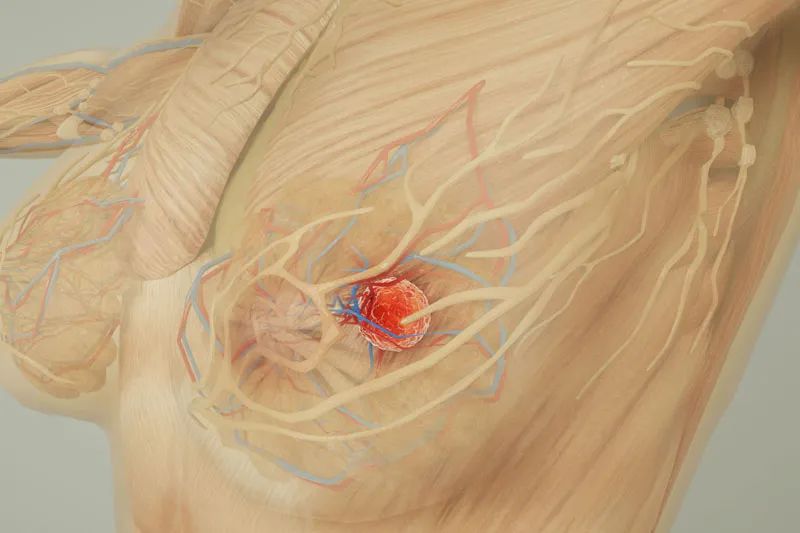
Metastasis is the process by which cancer cells break away from the area where they first formed (the primary site) and form new metastatic lesions in other areas or organs through the body's blood or lymphatic circulation system.
As there is no particularly effective way to stop this process, when a patient is diagnosed with a highly invasive type of breast cancer or advanced breast cancer, doctors need to treat not only the primary site, but also its metastatic potential.
Cancer cells use foot-like protrusions called "invasive feet" to degrade and break through the underlying tissue, thereby entering the bloodstream and metastasising in other organs. About four years ago, Dr Hava Gil-Henn and researchers from the Azrieli School of Medicine at Bar-Ilan University in Israel uncovered two important clues about the formation of the invasive foot.
When the cells were in the malignant stage, cellular levels of the proteins Pyk2 and dermatomyosin were suspiciously increased; but when the cells lost the ability to produce Pyk2, the researchers did not observe any metastases.
In a recent study, Dr Gil-Henn and Professor Jordan Chill of the Bar-Ilan Department of Chemistry described the interaction between these proteins and showed that this interaction is a prerequisite for the formation of metastasis in cancer cells. The team's findings were published in the journal Oncogene.
In the study, the researchers defined the precise fragment involved in the interaction between Pyk2 and dermatomyosin. They synthesised this small fragment, called a peptide, in the laboratory and administered it to mice carrying breast cancer. The synthesised peptide successfully competed with the natural Pyk2 protein for the 'attention' of dermatomyosin and essentially blocked the access of Pyk2 protein to dermatomyosin. This approach successfully inhibited the formation of the invasive foot and, as a result, the mice's lungs remained healthy with little to no metastasis.
"We are very excited to see how well this approach works," said study co-author Dr. Hava Gil-Henn. "This helps demonstrate the clinical potential of this therapy.
All of this was achieved using a small fraction of Pyk2, which leads to a reduction in lung metastases in a mouse model of breast cancer. In addition, it greatly reduced the invasiveness of breast tumour cells, preventing the maturation of the invasive foot in tumour cells and reducing the rate of actin polymerisation, which is required for invasive foot formation and progression. Together, all these findings provide clear evidence that this peptide actually prevents cancer metastasis.
Professor Jordan Chill, who specialises in the three-dimensional structure of proteins, joined the research team to determine exactly how the peptide prevented cancer metastasis.
"The process from the current study to the development of a successful drug would be very demanding and almost impossible to accomplish in this case without a structural view of the complex between the peptide and its target," said Professor Chill. Using an NMR experiment called NOESY, the researchers determined the position of each of the 881 atoms of dermatomyosin and 315 atoms of the peptide to create a three-dimensional image of the structure.
The spatial position of the atoms is the secret to understanding the strength of the bonds between proteins, which is crucial to creating drugs that effectively block such bonds. (It's like trying to unlock a world-class, ultra-complex lock and needing a custom-made key for it that fits tightly and cannot differ in any way.)
Dr Gil-Henn and Professor Chill are now focusing on converting peptides into better drug candidates. Different amino acid sequences are being tested to produce a product that will be more efficacious, more specific and a better fit for the therapeutic target. This is crucial because the site in dermatomyosin where binding occurs (called SH3) is similar to the SH3 site in other proteins, and the slightest slip-up and inadvertent binding to other proteins could lead to harmful side effects.

The combination of cell biology (which discovered the relationship between these two proteins and showed that we could effectively stop metastasis) and structural biology (which provided us with this binding method) has brought science closer to breast cancer treatment in a way that has never been done before. The researchers hope that this advance will lead to the development of new drugs that inhibit the formation of breast cancer metastases and will be a new option that can be used to improve the chances of survival and quality of life for patients diagnosed with invasive breast cancer and other metastatic cancers.
-
![]()
![]() MedicalFeb 26, 2026
MedicalFeb 26, 2026New Treatment For Uroepithelial Carcinoma Receives Fda Fast Track Designation With Encouraging Initial Results
-
![]()
![]() MedicalFeb 25, 2026
MedicalFeb 25, 2026Why excimer laser can treat myopia
-
![]()
![]() MedicalFeb 24, 2026
MedicalFeb 24, 2026New Treatment For Lung Cancer Approved! Significantly Better Than Chemotherapy!
-
![]()
![]() MedicalFeb 23, 2026
MedicalFeb 23, 2026Important Target For Novel NK Immune Checkpoint Inhibitor Identified!
-
![]()
![]() MedicalFeb 22, 2026
MedicalFeb 22, 2026Living cell anti-aging therapy is quietly emerging